Energy
Exploration Pages:
 Photosynthesis!
Photosynthesis!
 Energy Flows Through Life
Energy Flows Through Life
Other FT Exploring Pages
 What
is Engineering, Really What
is Engineering, Really
 Dr
Galapagos Dr
Galapagos
 SITE MAP SITE MAP
 Link
Pages Link
Pages
 Contact
Me Contact
Me
|
|
| |
The
Second Law of Thermodynamics
Or Energy
is Forever, but Not Exactly
 |
 |
 |
 |
| |
|
Keeping
It Simple (and Clear)
Teachers
and Learners:
The Second Law of Thermodynamics is probably the most misunderstood principle of physics.
Because of the confusion and pervasive misinformation regarding
this principle, I've dragged my feet shamelessly when it
came to dealing with it in this website. Well, I can't undo all the misinformation with one
little web site. So I won't try. If you are a beginner start here; and if you are not going to be an engineer or scientist, stay here. It
is probably all you will need to understand the basic energy change results the 2nd law predicts. Your head won't get filled with confusing non-thermodynamic and incorrect analogies.
We don't need the silly, and wrong, examples of messy desks.
Such things are not predicted by the 2nd Law of Thermo. Honest.
Other than the next few paragraphs, we won't discuss entropy on this page (but someday its page will come). Thermodynamic entropy is a measurable property of matter, not a vague predictor of universal decay. We don't need to discuss measurements of
disorder. We never need disorder. What are those units of
disorder anyway?
Disorderites? Messyisms?
Gobbledygooks?
Being a physical property, entropy has units. The units are energy divided by absolute temperature (We are talking about classical thermodynamics here - not getting into statistical thermo).
I don't believe there are units of disorder in any field, though it sounds like it could be a good legal term for court room judges ("You are guilty of generating 3.5 units of disorder in my court room").
All of the various 2nd Law definitions listed in text books
result from the basic energy change results I describe in
these pages.
But why start backwards? Start with the basic energy changes
the 2nd Law describes. Very simple.
Read the following at least 3 times:
It is only about energy.
It is only about energy changes.
It is only about the condition of the energy before
and after the change.
To be sure, there are interesting concepts about organizational
disorder, probability, complexity, and things getting messy (and the propagation of
misinformation about thermodynamic entropy). But it is incorrect to create metaphors and anlogies from thermodynamic entropy to explain those concepts,
and it only misleads beginners.
Relax, you don't need them to explain this concept to students.
The Second Law of Thermodynamics
absolutely does NOT say everything tends toward disorder (or decay)!
It is not a universal law of messiness. It is only about energy changes. Isn't that nice? We can all relax. My messy desk and your wrinkled shirt are not predicted or measured by entropy formulas and the 2nd Law of thermodynamics.
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| |
|
|
|
| |
|
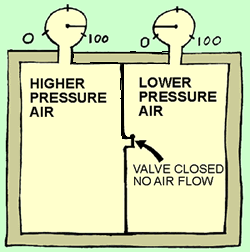
Picture
1: The pressure in the volume on the left is higher than the pressure
on the right. The pressure energy in the left side can be thought of as more "concentrated"
than the pressure energy on the right side. Both sides take up the exact same
amount of space (or volume), but there is more pressure energy in the left side.
More pressure energy in the same space means it is more concentrated.
|
|
 |
 |
 |
 |
| |
|
|
|
| |
|
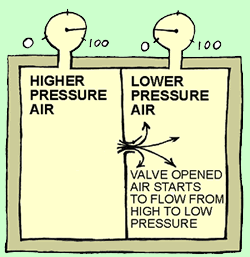
Picture
2: The
valve has just been opened. Immediately, air on the higher pressure left side
starts to flow to the right side, because the pressure is lower there. Just like
air escaping from a baloon.
Energy is flowing from more concentrated to less concentrated. |
|
 |
 |
 |
 |
| |
|
|
|
| |
|
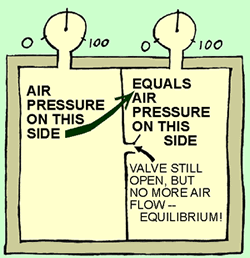
Picture
3: The air kept flowing through the opening until the pressure on both
sides was equal. See the pressure guages? They show the same pressure on both
sides. The pressures are now equal - no difference in concentration levels.
There is no more air flow through the opening.
We have reached equilibrium.
The total energy hasn't changed (First Law), but it is more "spread out"
or less concentrated now (2nd Law). |
|
 |
 |
 |
 |
| |
|
|
|
 |
 |
 |
 |
| |
|
|
|
| |
|
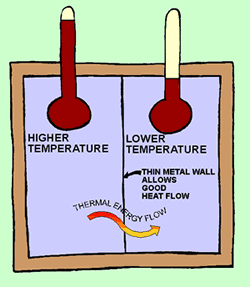
Picture
1: Two tanks of water. The water on the
left side is hotter than the water in the tank on the right side. There is only
a thin piece of sheet metal, or maybe some glass, separating the water, so heat
(thermal energy) can easily flow from one side to the other. Thermal Energy is
more concentrated in the hotter water. A cubic inch of water on the left side,
has more thermal energy in it than a cubic inch of water on the right side.
|
|
 |
 |
 |
 |
| |
|
|
|
| |
|
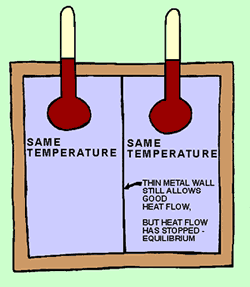
Picture
2: The thermal energy continued to flow from the left side into the
right side. The temperature on the right steadily increased, while the temperature
on the left side got steadily cooler. Eventually the temperatures on both sides
became the same, as shown by the cartoon thermometers above. Equilibrium
has been reached. The concentration of thermal energy is the same on both sides,
so there is no more energy flow. |
|
 |
 |
 |
 |
 |
 |
 |
 |
How
Everything Happens
 Energy makes everything
happen, and every time something happens, there is an energy change. There are two important natural "laws of energy" that describe what happens to the energy involved in every change. We call them "laws" because countless observations and
thousands of experiments have shown them to always predict what will happen. Energy makes everything
happen, and every time something happens, there is an energy change. There are two important natural "laws of energy" that describe what happens to the energy involved in every change. We call them "laws" because countless observations and
thousands of experiments have shown them to always predict what will happen.
 Ponder
that for a moment - how everything happens. It means we don't understand much,
if we don't understand both the first and second laws of Energy. Ponder
that for a moment - how everything happens. It means we don't understand much,
if we don't understand both the first and second laws of Energy.
These next few pages will give you an overview of the famous, but often misunderstood,
2nd Law.
Beyond the First Law
The First Law of Thermodynamics tells
us energy is conserved. The total amount never changes. But something does change.
I will call it "re-usability", for now. It's not an official text book word, but
pretty good for communicating the basic idea.
Remember that there has to be an energy
transfer for something to happen; energy changes form or moves from place
to place (heat flow, for example).
As energy moves and changes, the total amount of energy stays the same, constant
forever as far as we know.
That sounds good doesn't it?
Energy is forever.
But wait! If it's forever, why are all these do-gooders telling us we need to
conserve energy by using less? Can't we just keep using it over and over? Why
shouldn't everyone drive to work alone in a 300 horsepower
car?
The Rest of the Story...
Alas, my friends, there is always a rub, and when it comes to energy, the rub is described by the Second Law of Thermodynamics.
The first law would be quite happy to let us re-use energy over and over. The
first law is happy as long as energy is conserved. It's the happy law.
The second law may seem a little less happy to some. It describes the aftermath of every energy change that makes something happen. The second law says that each time energy gets
transferred or transformed, some of it, and eventually all of it, gets less useful.
That's the truth. It gets less useful, until finally, it becomes mostly useless (at least as far as its ability to make things happen is concerned).
All of the energy we use ends up, sooner or later, as what we engineers like to
call "low-grade" energy. This low-grade energy is only good for warming the air
around us a little bit. We can't use it to do things we consider useful, like generate electricity or make a car go. Inevitably, most of it gets radiated out into the vast cold universe,
lost to us forever.
To understand this, it is helpful to start with another aspect of
the Second Law. Let's call it "the direction energy moves" aspect.
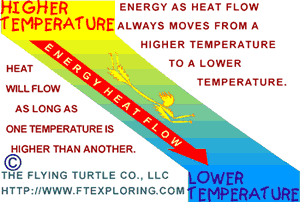
The Direction Energy Always Goes
The second law tells us which way energy naturally flows when not blocked or "pushed" by other mechanisms. It says energy has
an absolute unfailing tendency to go from "more concentrated" to
"less concentrated". It sort of "spreads out" and gets "diluted".
That's a good way for beginners to think about it.
Energy flows from a higher temperature to a lower temperature (heat flow).
Energy flows from a higher pressure to a lower pressure (expansion).
Energy flows from a higher voltage potential to a lower voltage potential (electric
current).
Energy flows from a higher gravitational potential to a lower gravitational potential
(falling objects).
Marbles and trucks roll downhill.
Water flows and falls from higher elevation to a lower elevation (downhill).
And last, but not least, chemical reactions proceed from higher concentrations
of molecular bond energy to lower bond energies.
In each of those cases, we can think of the energy in the higher level as being
more concentrated. Energy inevitably moves to a less and less concentrated condition.
Less concentrated = less useful.
For anything to happen, energy has to move or flow or change. Energy will keep
flowing or changing from a higher concentration to a lower concentration until
the concentrations are equal (not necessarily more disordered). We call that condition,
cleverly enough, equilibrium.
So that's one part of the Second Law of Thermodynamics. Energy will flow to a
more "spread out" or "less concentrated" condition. It stops flowing when there
is no longer a difference in concentration levels - when things have reached the
great state of equilibrium.
Simple enough, eh? Who needs a law for that?
The Unstoppable Tendency of Energy
Unless something is put in its way to stop it, or we utilize mechanical or biological machines to concentrate or store some of it for later use (see examples to the right and below), energy always flows that way. It
is an unstoppable tendency.
We can slow it down, or even stop it by blocking the more concentrated energy
from reaching or connecting with the less concentrated energy. But the potential
is still there. If we close the switch, open the valve, start the chemical reaction,
or break the levee, look out.
Potential Energy
That's a good way to think about potential energy, concentrated energy waiting
for its chance to flow to a less concentrated level. Things happen when energy
is allowed to move from high potential to low potential. Things stop happening,
or don't start, when there is equilibrium.
Examples
In the pressure tanks in the green column on the right, we can keep the valve
closed. As long as the high pressure side is blocked from the low pressure side,
nothing will happen. But the potential is there, waiting for its chance
to move, which in this case means opening the valve.
In the water tanks shown in the pictures to the right above, the thermal energy
in the hotter water will flow into the cooler water until the temperature in both
tanks is the same - equilibrium!
We can slow down the heat flow by adding a thick layer of insulation between the
two tanks. This will slow down the heat flow, but not stop it. Energy flowing
from hotter to cooler is unstoppable. Eventually, both sides will still reach
equilibrium and the temperatures will be equal.
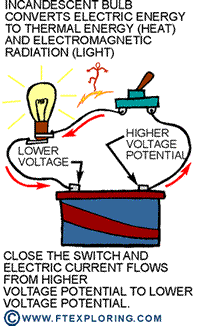
If we keep the light switch open, the electric current can't flow from higher
voltage potential to lower potential. But the tendency is always there as long
as the potential difference is there. Close the switch and electricity
flows from high voltage to low voltage, making the light bulb glow. The potential
energy is converted to thermal energy and light.
Hold a rock over your head. The gravitational potential energy is there. Let go
of the rock and it gets pulled down to the ground (or your head if you're not
careful). The rock's potential energy is converted to kinetic energy as
it rushes to get to the lower energy level of the ground. When it hits the ground
the kinetic energy is converted to sound waves and low-grade thermal energy, heating
up the rock and the ground.
We Can Catch Some Energy as it Flows from
High to Low Concentration
Nature and human engineers have learned to control and use energy
flows. We can catch some of it as it flows by, maybe with a turbine,
or piston and crankshaft, maybe even a sail or wind
turbine blades. We can transform some of it (but not all) into
useful work.
We Can Push Energy the Other Way
Despite Energy's unfailing tendency when left to its own inclinations to flow from more concentrated to less, we can also "push" or force some of it the other way, from less concentrated to more, such as when we lift a weight, compress a gas, pump water uphill, or, through photosynthesis, assemble molecules into energy storing glucose.
 |
 |
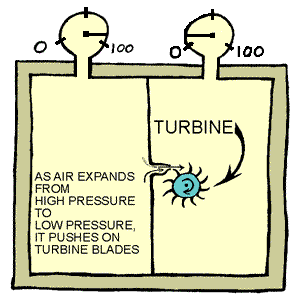 |
Catching
Some of the Energy:
The picture above shows how we might be able to convert some
of the concentrated pressure energy into mechanical energy.
The air has to push against the turbine blades as it flows
from higher pressure to lower pressure (expansion).
If the turbine shaft were attached to a little generator,
we could make some electricity. We could then use the electricity
to power an electric motor that would drive an air pump to
pump air back into the other side and start the cycle over
again.
Is this a perpetual motion machine?
|
 |
 |
 |
The
Big Picture and Perpetual Motion
In our turbine example to the right, we can "re-concentrate"
some of the energy that was stored in the left tank. If we connect
the turbine shaft to an electric generator we can make some electricity
while the expanding air is pushing the turbine blades around.
We could use an electric motor to drive an air pump to push the
air that flowed into the right tank back into the left tank.
Now energy is concentrated again as higher pressure in the left
tank and lower pressure in the right tank. Open the valve and let
the air expand into the right side, and once again it will spin
our little "turbine-generator" until the pressures in
both tanks are equal and the turbine stops.
Would this work? Can we re-use energy over and over?
No!
You must look at the big picture and follow all the energy transfers
involved in our system.
The first thing you would notice if you did this, and had an accurate
pressure gage, is that the pressure in the left tank would not be
as high the second time as it had been when you started. And the
third time you do it, the pressure on the left will be even lower...uh
oh, that's not a good trend. Our concentrated energy seems to be
getting less and less.
What's happening?
Each energy transfer in our system results in some of the energy
being turned into less concentrated, or low-grade, waste energy.
This inevitable result of each and every energy transfer is what
the 2nd law describes. Everytime anything happens, some of the energy
that caused that "happening", becomes less concentrated
(which means less available for useful work in this case), and you
can't get it back.
Due to air friction and turbulence, not all of the air-flow energy
passing through our turbine is converted into mechanical force on
the blades, some is just frittered away into useless random molecular
motion, or heat as
we often call it. The turbine is never 100% efficient.
That energy is lost to us. It's still around, but we can't use it.
The spinning shafts that support the turbine and generator rest
on bearings where, because of friction, some of the energy is turned
again into heat that
flows uselessly into the surroundings.
That energy is now also unavailable to us forever.
The generator itself, because of windage and electrical resistance
is not 100% efficient. Some more energy from these losses trickles
away into the surroundings as heat.
More energy lost.
Finally the pump itself is only about 70% efficient and there are
pressure losses in the flow passages leading to and from the pump.
When all is done, we won't get very much of the "available"
concentrated pressure energy that was originally in the left tank back
into it again.
Still more energy lost.
At each step of our process of "expansion=>power generation=>recompression",
some energy is lost. It "leaks" away into lower-concentration.
It becomes un-useable. For the purposes of our little system, and
most anything else, it is lost forever.
So even though we are able to keep re-concentrating energy in one
area, in the big picture it takes more energy to re-concentrate it than we get back. We always lose. The electricity made by
our little turbine isn't enough to keep the system going. It will
eventually "wind down" and stop, because all of the energy
will eventually become low-grade and useless. The only way to keep
it going is to replace the "lost" energy with some concentrated energy from outside the
system.
We will have to plug our electric air pump into an electrical outlet
to convert some of that concentrated electrical energy from the
power company into compressed air and heat. And the power company has to keep burning coal or gas to replace what we use.
The above describes why we can't have perpetual motion machines.
That's another one of the several ways the 2nd Law is sometimes defined.
"You can't have perpetual motion machines" we say, because
some energy in every process becomes low-grade and useless. You
have to keep adding fresh concentrated energy from outside the machine,
or the machine will eventually come to a stop.
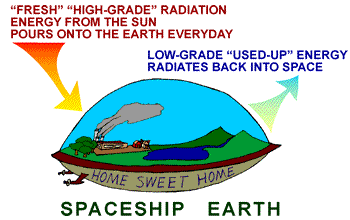
On earth our cycle of energy conversion
is pretty much this:
 Earth
is not a perpetual motion machine either. Each day concentrated
energy from the sun shines down onto the earth. Earth
is not a perpetual motion machine either. Each day concentrated
energy from the sun shines down onto the earth.
This light energy (electromagnetic radiation) powers almost all
life through the mechanism of photosynthesis.
The solar energy also causes wind to blow, rain to fall, and snow
to melt. During each of these activities, some of the solar energy
is converted to low-grade thermal energy...
Until....yup, you guessed it....all of the once concentrated solar
energy becomes too "spread out" and diluted to use. In
the end, it meekly radiates back out into the cold vastness of space.
From space it comes, to space it returns - but in a much less concentrated
form.
Without a new blast of high-concentration high-grade solar energy
everyday, the earth would quickly become lifeless and frozen.
 The
process of using energy always converts some of it into less and
less useful form. The
process of using energy always converts some of it into less and
less useful form.
It must be replenished, as fast as we use it, with new sources of
concentrated energy.
And that,
my fellow humans and friends
living on
and sort of sharing the energy on
our spaceship earth,
is the inescapable truth defined by the 2nd Law of Thermodynamics.
|
|
| |
If this page was helpful, please recommend it:
|
|
| |
|
|
 |
 |
 |
|
|
|