 |
 |
 |
| |

Kent
contemplates the mysteries of heat flow.
Where does the "hotness" go?
Will it be home for dinner?
Is there such a word as "hotness"? |
|
|
You Can't Stop the Heat Flow
Energy
is flowing into and out of your body, and everything else, all the time. I'm not
talking about energy from the food you eat as a card carrying member of the Food
Chain, though energy does get into animals in that way too.
Energy flows into your body from the surrounding air,
from surrounding objects, and from the sun when you're outside, or even light
bulbs when you're inside. Energy also flows out of your body into the surrounding
air, into the surrounding objects, and even into outer space, most notably if
you are outside on a clear night.
This type of energy transfer from one place to another
place is driven by differences in temperature and is called heat flow. It is a
really big deal - one of the major driving forces of nature (if you're interested
in major driving forces - and who isn't? - not to be confused with Tiger Woods
or Barry Bonds).
Heat
flow from the sun to the earth is the force that makes weather. The mother of
all earthly driving forces.
|
|
|
| You
can't use heat flowing into your body as energy like you can use the energy in
food. But it does help to keep you warm - or too warm. Animals in cold climates
don't have to burn as many calories in the summer as in the winter. How come?
|
| |
 |
| This
man had to cover himself with longjohns and many other layers in order to stay
warm while his active daring children ice skated and kept warm with hardly any
layers because they were generating a lot more internal energy by exercising while
he stood around like a sissy. |
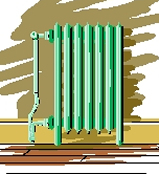 |
| These
great old radiators used a combination of radiation and conduction
to heat a room. Down in the basement was usually a steam boiler converting the
chemical energy stored in coal (in the old days) or fuel oil or natural gas (more
common nowadays), into "heat" which was used to convert liquid water
into gas phase water (steam to you laymen). The steam flowed up into the radiators
where its heat was transferred by conduction to the air and by radiation to the
objects in the room. |
|
|
|
| |
|
Heat flow from the sun to the plants and algae and some types of bacteria is the energizer
of photosynthesis - the provider of food for almost all living things.
For animals heat flow is a really big deal too.
The way that each animal is made and behaves and where they can live, has a lot
to do with heat flow.
Because of heat flow, cold-blooded reptiles and
insects that live in cold climates only come out in the summer. Heat flow is why
there are a lot more reptiles and insects in equatorial climates than arctic climates.
 Warm-blooded mammals and birds with their high metabolisms
and hotter bodies can live and move in some pretty cold places. But, because of
heat flow, they have to have good dry fur or feathers, or a good layer of blubber,
and plenty of high energy food to keep that body heat coming.
Warm-blooded mammals and birds with their high metabolisms
and hotter bodies can live and move in some pretty cold places. But, because of
heat flow, they have to have good dry fur or feathers, or a good layer of blubber,
and plenty of high energy food to keep that body heat coming.
When you put on a jacket in the winter, or wear
shorts on a hot day, you are adjusting your heat flow, trying to help your body
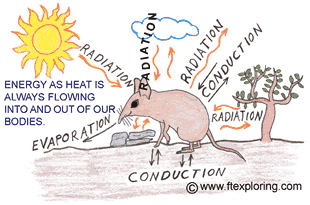
keep its temperature very close to 38 degrees C (98.6 deg. F). It's a never ending
struggle. Energy as heat is always flowing out of, and into, your body.
It's dangerous to be too hot and dangerous to be
too cold. Just like Goldilock's porridge, our body temperature has to be "juuust
right".
Animals and human engineers use all kinds of clever
ways to transfer heat. Elephant ears act like giant radiators to transfer heat
out of their bodies. Car radiators are used to transfer heat out of the car engine.
There are countless fascinating examples. In pages yet to come we will explore
some fascinating examples of how animals and human made machines use the same
basic principles of heat flow, along with some pretty clever techniques, to keep
their operating temperatures "juuust right".
But first we must learn three simple things about
how energy heat flow works.
|
|
|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|
| |
|
|
|
|
| |
|
Which
Way Does It Go and How Fast Does it Go?
What
happens when you put a cup of hot liquid on the counter in the kitchen?
(Confused pause while you wonder if this is a trick
question.) Everybody knows that if a cup of hot water sits in a room,
the water in the cup will gradually get less hot.
Assuming, of course, that the air in the room is at a temperature that humans
find comfortable, and that gravity holds the water in the cup and the cup on the
counter.
|
|
|
| |

| Kent
demonstrates how not to determine if the water is hot. |
 |
|
But what really happened to the water in the cup
on the counter? How did the "hotness" escape? Where did
the hotness go? Why did it go? It never stays for dinner. Didn't it
like us? What is "hotness" and "coldness"? Are
those real words? And why do I keep losing socks?
Here's some more questions for you. What
temperature will the hot chocolate cool down to? Why doesn't it just
keep getting colder and colder until it freezes? It seems ridiculously
obvious that the hot water won't freeze, but when we are asked to explain it,
most of us go, "uh...". Or we resort to the cowardly response,
"That's just the way it is". Or the truly brave approach,
"I haven't got a clue".
In this page, and others, we'll talk about where
the "hotness" went. There are three fundamental things about
heat flow that will answer the above questions (except for the ones about where
my socks go). |
 |
 |
 |
 |
|
|
|
| |
|
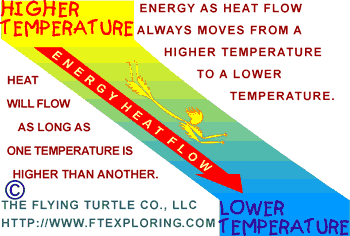 The
Three Things To Know The
Three Things To Know
Here are three easy things to know about the way heat flows:
1) There has to be a temperature difference. Energy only flows as heat
if there is a temperature difference.
2) Energy as heat flows from a higher temperature to a lower temperature.
3) The greater or larger the difference in temperature, the faster
the energy flows.
(See The Second Law of Thermo Pages)
|
|
|
| |
|
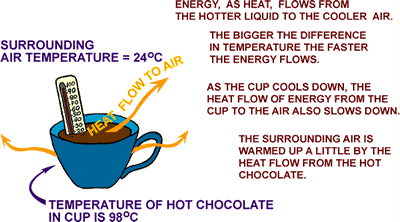 |
Let's
consider a cup of hot chocolate in the light of our new found wisdom.
The cup will only cool down if the air surrounding the
cup is cooler than the hot chocolate. The energy doesn't disappear. It flows by
conduction into the air around the cup and the counter top it is sitting on. It
also travels by radiation into the cooler surfaces around the cup, like walls
and cupboards.
The cup cooled down, the room heated up.
That's right. The air in the room heated up.
|
|
|
|
| |
|
You
didn't notice it because the large room full of air can easily absorb that amount
of added heat energy without much change in temperature.
But if you had a thermometer that could measure
temperature to very small fractions of a degree and if you could make the kitchen
airtight so that no air could flow in or out, then you would be able to measure
a slight increase in the temperature of the kitchen air. And if you put hundreds
of cups of hot chocolate in the kitchen it might get pretty darn hot and muggy
in there.
According to the First
Law of Thermodynamics, the energy that flowed into the air and the other
surfaces in the room exactly equals the energy that flowed out of the cup. It
has to be that way because energy can't just disappear into nothingness. The total
amount of energy never changes.
 The
energy in the cup moves, as it always does, from the hotter "stuff"
to the cooler "stuff". The hot stuff has more energy in its atoms
than the stuff that is cooler. The higher energy atoms transfer their energy to
the lower energy atoms. The
energy in the cup moves, as it always does, from the hotter "stuff"
to the cooler "stuff". The hot stuff has more energy in its atoms
than the stuff that is cooler. The higher energy atoms transfer their energy to
the lower energy atoms.
The hot chocolate will cool down until it becomes
the same temperature as the surrounding air.
(Check out 2nd Law of Thermo)
The stuff below
assumes you've had a little physics, maybe highschool or older (or are smarter
than I was in junior high):
Heat Flow as a Rate
Units of Heat, Energy, Heat Flow, & Power
Heat flow is energy moving. It has the same units
as power - energy per unit time - or energy/time. It means that during the given
amount of time, during which heat is flowing, a certain amount of energy is transferred
or moved from one place to another place. Confused? Let's make up an energy unit.
Anyone can do it.
Let's pretend units of energy are measured in wompy
gloopers (WG). Let's also say that 60 wompy gloopers of energy flows from an average
adult human male body to the surrounding air (temp. at about 24 degC) during every
minute (not a very exact definition). Then the rate of heat flow is 60 wompy gloopers
per minute, or 60 WG's/min. See? Units of energy per unit of time. How about in
seconds?
Yup, 60 WG/minute equals 1 WG per second
or 1 WG/sec (60 divided by 60).
What is the heat rate in hours? There are 60 minutes in an hour, so 60 WG/min
equals 3600 WG's/hour (60 times 60). I am an average adult human male. As I write
this, heat energy in my cells, generated by the metabolism of sugar to energy
during cellular respiration, is flowing out of my body into the room at the rate
of 3600 WG/hr.
But alas, nobody is measuring heat flow energy in
wompy gloopers. You might be more familiar with units like joules or calories
or kilowatt-hours (Kw-hr), or even BTU's (British Thermal Units), an old favorite
of mine.
One joule (J) is the amount of energy generated
when a force of 1 newton (N) is exerted over a distance of 1 meter (m). So a joule
has the units of newtons times meters, or N-m. A joule is the official unit for
energy, heat, and work, in the International System (SI). Heat flow is measured
in joules per second. Most of us are more familiar with watts as the unit of heat
flow rate and power. Watts are a measure of power, not energy. Power is a measure
of how fast energy is "happening". One watt means that 1 Joule of energy
is being transferred every second,
or 1 J/s. A killowatt (Kw) is 1000 joules per second or 1000 watts.
A typical adult male human being, sitting around
in a room of normal temperature, not doing much (hopefully reading rather than
watching TV), generates about 100 watts of heat flow into the air surrounding
him. From my WG definition above, we can say that 60 wompy gloopers equals about
100 watts. So 1 WG = 1.67 W (100 divided by 60). Just like that, we invented a
new unit of energy.
What happens if the furnace breaks and the air temperature
in the house gets colder?
In the first case the air temperature was 24 degC and
my body temperature was at the normal 38 degC then the temperature difference
was 14 degC (38 minus 24). So at a temperature difference of 14 degrees C, energy
flows out of my body into the surroundings at 100 Watts or 60 WGs.
After a few hours with a broken furnace the air
temperature in my house falls to 0 degrees C (It's cold outside. This is Wisconsin).
Now the temperature difference between my body and the air is 38 degrees C. If
I don't have enough sense to put on more clothes, or wrap myself in a few blankets
(insulation - which is a subject for another page), I am going to feel very uncomfortable.
How come? Because as we know from our discussion above, the temperature difference
has become larger, meaning heat will flow much faster from my body into the surrounding
air
.
If the heat flow rate from my body is faster than the
internal energy produced by my metabolism, I will start to feel very uncomfortable.
My body temperature will start to drop because I cannot produce internal
energy as fast as it is flowing out of my body. In addition to being miserable,
I will be in danger of hypothermia. Hypothermia is what they call it when your
body temperature gets lower than approximately 38 deg C (98.6 degF). A degree
or two lower is no big thing occasionally, but if it gets much more than that
for very long, I could be in trouble. Ah, the joys of heat flow.
If this page was helpful, please share:

|
|
|
There
are two basic ways that energy can be transferred from one place to another through
the heat flow process - radiation and conduction.
Radiation is a type of energy that can travel
through space. It doesn't need matter to conduct it from place to place. It can
travel through a vacuum, no trouble. It can also travel through air, no trouble.
| When
you stand near hot molten lava, the heat you feel on your skin is mostly radiant
heat. This type of heat doesn't need air to travel through. Even if you were standing
in a vacuum (no air) you would feel the heat (except you'd be unconscious, or
worse, from lack of air). |
|
 |
| |
The
sun is a big hot ball that radiates energy into space. A tiny portion of the sun's
energy "hits" the earth. |
| |
 |
 |
 |
 |
Almost all of the energy from the sun that travels 93 million miles in 8 and 1/2
minutes through the vacuum of space is radiant energy. When you stand outside
on a sunny day feeling the warm rays, remember that only 8 and 1/2 minutes ago
it left the sun. Scientists call it electromagnetic radiation. Infrared,
ultraviolet, and visible light are examples of electromagnetic radiation
that can transfer energy from one object to another object.
 Believe it or not, your body and all other objects are always giving off or absorbing
heat by radiation. Heat transfer by radiation goes from a hotter object to a cooler
object - like from the sun to earth, or from hot coals to you, or from your body
to the cold walls of a lonely castle on a dark and stormy night.
Believe it or not, your body and all other objects are always giving off or absorbing
heat by radiation. Heat transfer by radiation goes from a hotter object to a cooler
object - like from the sun to earth, or from hot coals to you, or from your body
to the cold walls of a lonely castle on a dark and stormy night.
Conduction is the type of heat flow that
results when things are actually touching. Energy traveling as heat by conduction
needs matter to flow through. If you touch a hot object the heat is conducted
by physical contact with your skin. The energized atoms in the object transmit
their energy to the atoms in your hand. If you are standing in cold air, the heat
from your body flows from the molecules in your body into the cold air molecules
that are touching your body. If you are floating in cold water, the heat flows
from your body into the cold water molecules that are touching your skin. If you
fry vegetables on a stove you are relying on conduction to cook your vegetables.
Heat from the flame flows through the metal by conduction, into and through the
cooking oil by conduction, and into and throughout the vegetables by conduction.
Conduction cannot travel through a vacuum because
in a vacuum there are no atoms or molecules making contacting with other atoms
or molecules. Something made of atoms or molecules has to touch something else
made of atoms or molecules in order for there to be conduction.
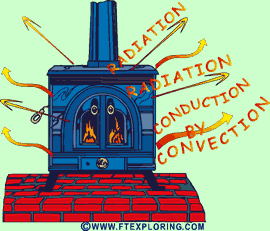 |
Heat
from a wood stove is radiated to cooler surfaces like walls, floors, ceilings,
furniture, and people.
Energy as heat is also conducted into the air and circulated naturally
by a process called convection from the hot metal surfaces to the air surrounding
the stove. |
Power vs Energy
Some of us get power
and energy mixed up. We shouldn't. It's easy. Here are some vague explanations
to make it clearer:
Energy, as you know, is that certain something that makes
things happen. The more of it you have, the more you can make happen. The
chemical energy stored in 10 gallons of gasoline (petrol) will move your car a
lot further than 1 gallon of gasoline.
Power measures or describes how fast those energy things happen. It is a measure
of how fast energy is being converted from one form to another.
A unit or measure of power is the Watt.
A 100 Watt light bulb uses 100 joules of energy every second, because 1 Watt =
1 Joule per second. Every second that light bulb is converting 100 joules of electrical
energy to 100 joules of heat and light (mostly heat - that's why it burns to touch
one).
Another well known unit of power is the horsepower.
It is based on a lesser known unit of energy called the foot pound force (ft-lbf).
If you move something by pushing on it with a 1 pound force for a distance of
1 foot, you have done 1 foot pound of energy.
Someone once actually hooked some horses to some weights and pulleys and decided
that the average horse could generate about 550 foot-pounds of work
every second. Energy divided by time again. That is power. He decided to call
that 1 horsepower.
So 1 hp equals 550 foot pounds of energy per second. Every second that poor horse
is lifting 550 pounds through a distance of 1 foot, or 1 pound through a distance
of 550 feet, or 5.5 pounds through a distance of 100 feet, or 55 pounds through
a distance of 10 feet. You try it sometime.
|
|
 |
 |
 |
 |
|
|
|
| |
|
|
|
|
| |
|
Top of the Page |
|
|
| |
|
|
|
|
 |
 |
 |
 |
 |
|
|



