|
Top
PHOTOSYNTHESIS - TURNING SUNLIGHT INTO LIFE. SCIENCE &
TECHNOLOGY EDUCATION FROM FT EXPLORING.
|
|
|



|
INTRODUCTION TO PHOTOSYNTHESIS
Photosynthesis basics; Making sugar out
of air, water, and sunshine; Carbohydrates; What's
a carbon-based life form; Oxygen, carbon, and other things we're
made of; What a glucose
molecule looks like - sort of. Science
education from FT Exploring.
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| |
Photosynthesis, Energy, and Life - Page 3 |
|
 |
 |
 |
 |
 |
|
|
| |

| |
Energy moves through the food chain from life form to life
form. The first step is always photosynthesis in which the sun's
radiant energy, that pours onto the earth everyday, is turned into carbohydrate
molecules. These carbohydrates are used by all living things as fuel for
energy, and as building blocks to build more pieces of themselves.
|
|
 |
 |
 |
|
| What
Plants Do With Sunlight |
| |
 It's all powered by the sun!
It's all powered by the sun!
All those plants and animals - swooping, running, hunting,
growing, flying, swimming, sneaking, eating, sniffing, digging, buzzing, chirping,
biting, stinging, reproducing, chewing, licking...well, you get the idea....
It's the Mysterious
Everything flowing through it all, through every single one of
us! It comes into the food chain as radiant energy from the sun, makes
everything happen, get's "used up", and goes out. (The
energy doesn't really get used up, it just turns into a form of energy that most
living organisms can't use for food - heat.)
|
 |
 |
|
|
|
| |
What Goes In Doesn't Come Out - Or Does It?
|
 |
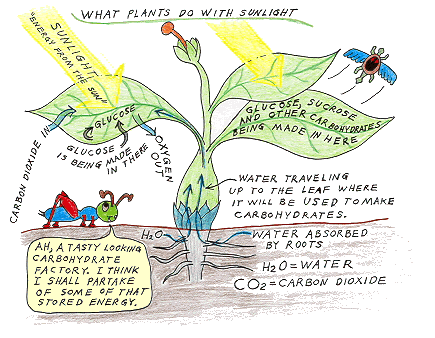
Almost every green surface on a plant is full of cells that are working away making
sugar while the sun is shining.
The process is very complicated with lots and lots
of steps, but the basics are very simple. Below is a simplified
summary of what happens.
Ingredients:
Take some carbon dioxide molecules (CO2)
out of the air (if you are a dry land plant). Take some water molecules
(H2O) from the plant's water supply, and add a little energy from sunlight.
Instructions:
Break apart the water molecules into Oxygen atoms (O),
Hydrogen protons and electrons. Now add the Hydrogen to the Carbon and Oxygen
in CO2 (be carefull to always maintain a ratio of one carbon and one
oxygen for each two Hydrogen) and voila, you have new molecules of very useful
carbohydrates.
Leftovers:
Take the leftover oxygen atoms, combine them into groups
of two (they don't like to be alone), and put them back into the air as oxygen
molecules (O2).
So what went in did come out? Sort of. Didn't it? |
|
| |
This power for life (and everything else), that we call energy,
flows into the food chain through our friends the busy plants. The plants do something with that seemingly nothingless energy that seems miraculous. They
turn it into food. This is very nice of the plants because animals can't eat sunshine.
They can only eat plants or each other.
plants do something with that seemingly nothingless energy that seems miraculous. They
turn it into food. This is very nice of the plants because animals can't eat sunshine.
They can only eat plants or each other.
Scroll down to the section below or click on this
link for a look at a very important carbohydrate
molecule.
(You
ought to know the word for the process they use to make all this food. The
word is Photosynthesis. It's not that big of a word really. And only
hard to say if you have a mouth full of crackers. Let's practice. Say it
together, "foto-sin-the-sis" The "th" in the "the"
is pronounced like the "th" in thistle or theory, not like the "th"
in the or this. See? Ain't English great!) |
|
 |
 |
 |
|
|
 |
 |
 |
 |
 |
 |
 |
|
|

|
| |
| |
It
has been estimated that 100 billion tons of sugar (sugar is a simple carbohydrate)
is made every year by green plants, marine algae, and certain kinds
of bacteria. That is equal to the weight of 666 million blue whales
(give or take a few hundred thousand)!
The certain kind of bacteria is more properly called
cyanobacteria. ! |
|
 |
 |
 |
|
| |
Gasoline
is fuel for cars. Carbohydrates, made only by plants during photosynthesis, are
the fuel for all living things.
A very important carbohydrate is a sugar (yes
sugar is a carbohydrate) called glucose.
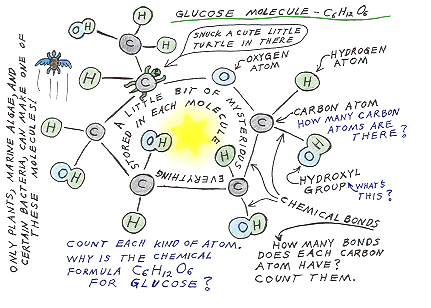
Glucose is the basic fuel and basic building material for much of life.
The picture below shows how glucose molecules are
constructed. Notice that they are made up only of Carbon (C), Hydrogen
(H), and Oxygen (O).
It's not as complicated as it looks. Look at it
for awhile. Count each type of atom. How many carbon atoms are there? |
 |
 |
|
|
|
| |
Glucose and other sugars (all made during photosynthesis) are the fuels
that power all living things. While you are sitting there reading this, every
cell in your body is busy "burning" sugar to provide the energy for
you to think and breathe and digest food and pump blood and stay warm and say
things like, "Wow, this is interesting!"
But sugar is also a building block out of
which is made the cells of our bodies and the bodies of all other living things.
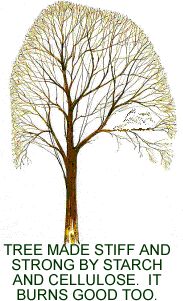
For example, the walls of plant cells are made in
large part of starch and cellulose. They are the structural material
of leaves, stems, limbs, and trunks. Starch and cellulose are made of long chains
of glucose molecules attached together.
Sugar molecules are also used as starting material
for molecules like the amino acids and nucleotides from which larger more complicated
molecules used by our bodies are formed. |
 |
| |
In
the picture above, the little black lines between atoms represent the chemical
bonds which you can think of as sort of like hand holding between the atoms.
It's how they hold on to each other. It's not really how they look. There aren't
really little black lines between the atoms. For that matter, there isn't really
a little glowing yellow star of energy in the center of each molecule. The
stored energy is really in the chemical bonds. When those bonds are broken the
stored energy is released.
Many carbohydrate molecules have twice as many hydrogen
atoms as carbon and oxygen. If we've drawn it correctly, the one above should
have 12 Hydrogen (H), 6 Carbon (C), and 6 Oxygen (O). See?
Carbon atoms have a quirk. They always want
to hold on to four other atoms or groups of atoms (molecules). (Go ahead, count
them in the picture above.) This ability to hold on to four other atoms, allows
for a tremendous diversity and variety of molecules based on the carbon atom attached
to other atoms. Pretty much all living things are built around carbon based molecules.
The study of the chemistry and molecules of living things
is basically the study of the chemistry of carbon. Living compounds are based
on carbon atoms attaching to themselves and to other atoms.
That's why in futuristic science fiction movies
the evil machines and computers are always trying to eliminate the "carbon-based"
life forms that are infesting the world. That's us! |
 |
 |
|
|
| |
| |
Oxygen
is not just used by our bodies for respiration.
It's not just in the air.
It's the most abundant element on earth. Half of the weight of our entire planet
is oxygen. About 89 percent of the total weight of the ocean is oxygen. Most rocks
are about half oxygen by weight. And about 65 percent of the weight of your
body is oxygen.
Of course, a great deal of that oxygen in our bodies
is in water which has one oxygen atom and two hydrogen atoms in each molecule.
But it is also a key ingredient of many other molecules. |
|
 |
 |
 |
|
| |
Your whole body is mostly made of just 6 different types of 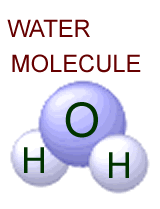 atoms - carbon, hydrogen, oxygen, nitrogen, sulfur,
and phosphorous. There are, of course, a few other minerals here and there,
but the above elements make up about 98 percent of the human body. Those
6 atoms combine themselves with each other (always making four attachments to
carbon) to make all the carbohydrates, proteins, fats, and nucleic acids, that
make up the human body and all other life forms.
atoms - carbon, hydrogen, oxygen, nitrogen, sulfur,
and phosphorous. There are, of course, a few other minerals here and there,
but the above elements make up about 98 percent of the human body. Those
6 atoms combine themselves with each other (always making four attachments to
carbon) to make all the carbohydrates, proteins, fats, and nucleic acids, that
make up the human body and all other life forms.
Phosporous and sulfur are each less than 1 percent
(though that 1% makes a big difference) so almost 97 percent is made up of the
big four - carbon 19.4%, hydrogen 9.3%, nitrogen 5.1%, and most of all,
oxygen with 62.8%. |
 |
 |
|
|
|
| |
|
|
|
|
 |
 |
 |
 |
 |
 |
 |
|
|
|
|
|
©
Copyright 2009, 2014.
David E. Watson. All rights reserved. Everything
in the Flying Turtle web site is copyrighted. For information
concerning use of this material, click on the word Copyright.
|
|
|
|
| |
|
|
 |
 |
 |



